Upgrading to Natural Refrigerants Systems
(CO2, Propane, Glycol, Ammonia)
(CO2, Propane, Glycol, Ammonia)
The current landscape confronting supermarkets and other food retailers, as well as industrial refrigeration system operators, and the need to move to natural refrigerants is in some ways more challenging than ever.
How Refrigeration Systems Work
To understand why this transformation has occurred it’s helpful to have some basic understanding of how refrigeration works. To most people refrigeration is simply the process of making things cold, or even frozen for which refrigeration is also needed. But what is more accurately happening when refrigeration takes place, is that warmer air is removed from one place such as in a refrigerator, food display cases or walk-in freezers, where it is objectionable, and transferred to other places such as to outside a store where it is otherwise not objectionable. Refrigeration systems achieve this effect through a combination of physics and machinery.
The physics aspect of the process has to do with the fact that heat is a form of energy. The law of conservation of energy—also called the first law of thermodynamics—states that energy can neither be created nor destroyed. It can only be changed from one form to another such as from heat to light or chemical to electrical energy. In practical terms, and also with regards to refrigeration, this means it can be moved but not eliminated.
The mechanical aspect of refrigeration relies on certain components to accomplish this movement of heat from one place to another. The four basic parts of a refrigeration system (i.e., the machinery) are:
- the compressor
- the condenser
- evaporator
- and a metering device (typically an expansion valve).
These components, along with a substance (refrigerant which absorbs heat) and some other elements, work together to transfer heat from one place to another.
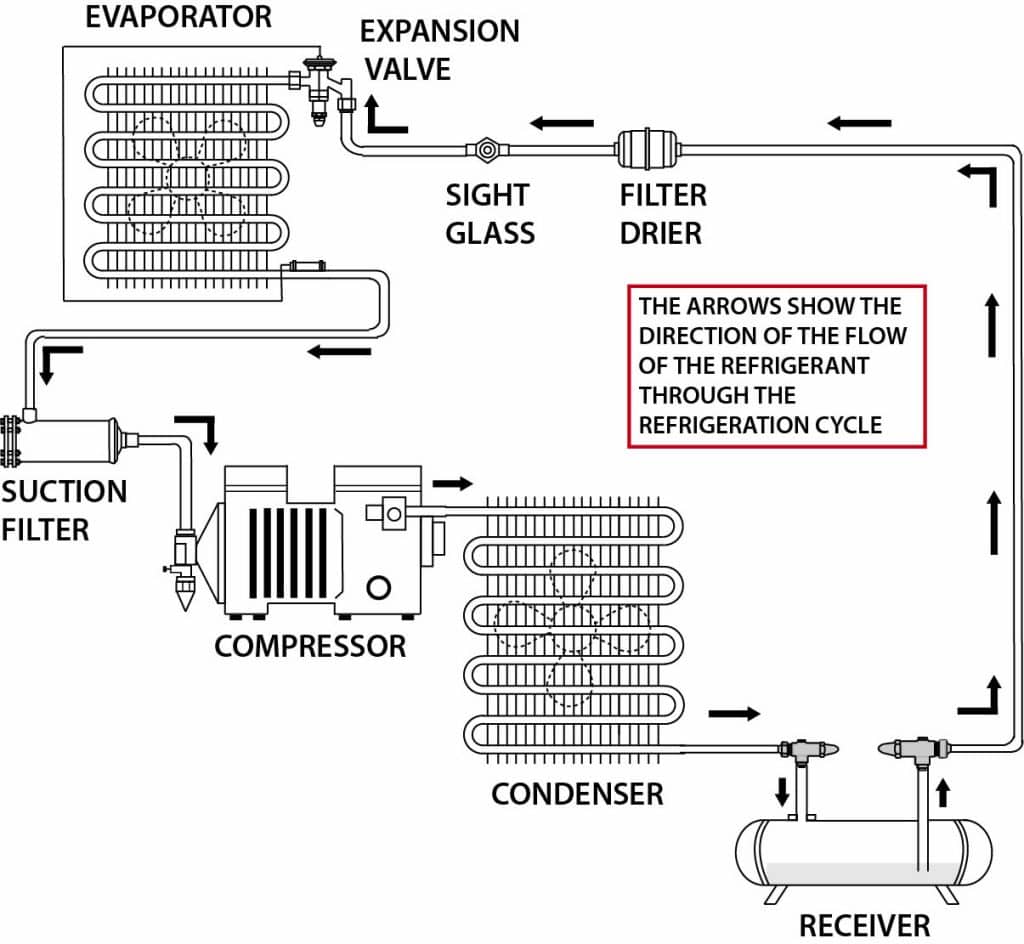
Basic Refrigeration Diagram
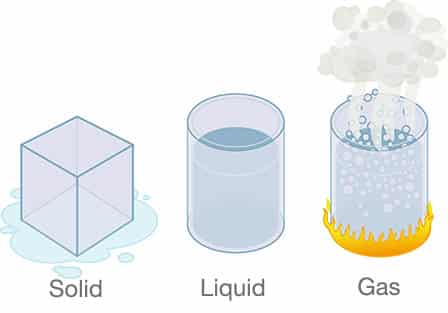
One way to think of this process is to focus on the two parts of the system that are called heat exchangers, the evaporator and the condenser. The evaporator takes away heat through evaporation, as its name implies, and likewise the condenser gives off heat through condensation. The latter is what happens when water freezes and the former is what happens in the evaporator, and in fact that’s what the refrigerant does as it moves through the evaporator—it boils. In both cases this takes place through a physical transformation called a state change where a liquid is turned into a gas and vice versa. Matter generally exists in one of three states: solid, liquid and gas. A combination of temperature and pressure determines the state of a substance which at a molecular level has to do with how much the atoms of a substance move around. Atoms move very little in solids, more so in liquids, and most of all in gases. Boiling a pot of water that turns into steam when it reaches a temperature of 212°F is a familiar example of this phenomenon in one direction. Freezing that same pot of water is of course has the opposite effect once its temperature is reduced to 32°F (0° Celsius).
Change of State
In refrigeration systems, this process is employed through the use of a substance that under normal pressures and temperatures exists as a gas and in the case of refrigerants is often referred to as a gas. The metering device (usually an expansion valve), previously noted, a s its name implies expands the gas so that when it enters the evaporator, it absorbs heat. Evaporators (and typically expansion valves) are found in the food display cases, walk-in coolers and freezers in grocery stores and anywhere else refrigeration is used to remove heat. From the evaporator, the gas through the pumping action of the next component in the system, enters that component which is a compressor.
s its name implies expands the gas so that when it enters the evaporator, it absorbs heat. Evaporators (and typically expansion valves) are found in the food display cases, walk-in coolers and freezers in grocery stores and anywhere else refrigeration is used to remove heat. From the evaporator, the gas through the pumping action of the next component in the system, enters that component which is a compressor.
Doing exactly what its name implies, the compressor compresses the gas. The gas enters the compressor as a low temperature, low-pressure vapor (another term for a gas). The compressor through its action produces a high temperature, high-pressure vapor.
From the compressor the refrigerant in the form of a superheated vapor then flows to the condenser. There, the refrigerant initially changes to a saturated state that contains both liquid and vapor as a result of it releasing heat through the condenser. By the time it exits the condenser, the refrigerant has entirely returned to a liquid state due to the amount of heat it has given off as it flows through the condenser. Coiled tubing inside of the condenser through which the refrigerant moves is commonly referred to as coils due to its “coiled” shape in the condenser. The same arrangement that accomplishes the opposite transformation in the evaporator is also often referred to as a coil.
By the time the refrigerant leaves the condenser, with most of its heat released (rejected) to the outside cooler air, it has almost entirely changed to a liquid. From there, the still high pressure, high-temperature refrigerant moves back to the first component in the system (already mentioned), the expansion valve and the process continues in what is referred to as the refrigeration cycle.
Other parts are typically found in modern refrigeration systems, but the evaporator, compressor, condenser and expansion valve are the four essential ones common to all refrigeration systems. And of course, along with them, refrigerants are an indispensable element of the process. They are also, as such, the object of successive and continuing regulatory scrutiny over more than the past 30 years.
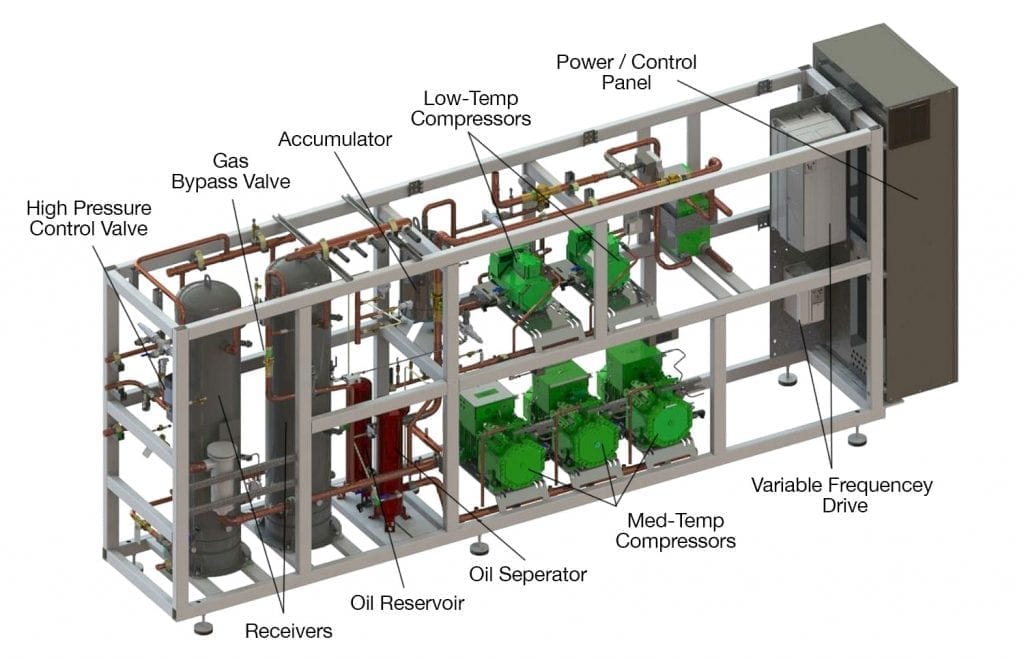
Sample R744 Refrigeration System Rack
Refrigeration is a Gas
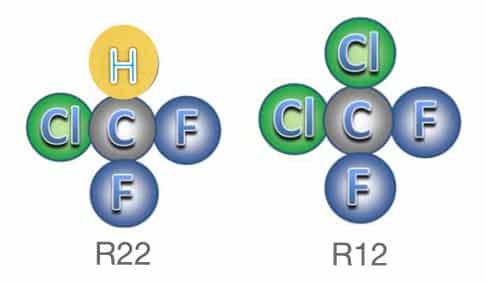
 Throughout most of the history of commercial refrigeration, the industry has primarily relied on synthetic refrigerants. But beginning in 1987, the Montreal Protocol set out a pattern for identifying and then attempting to regulate and eventually curtail the use of these substances that were found to be environmentally harmful. One, in particular, that was the most widely used refrigerant at the time (R12, Freon-12 or Freon for short), was found to be a threat to the ozone layer which protects life from the harmful effects of the sun’s ultraviolet radiation, and even more so a contributor to global warming.
Throughout most of the history of commercial refrigeration, the industry has primarily relied on synthetic refrigerants. But beginning in 1987, the Montreal Protocol set out a pattern for identifying and then attempting to regulate and eventually curtail the use of these substances that were found to be environmentally harmful. One, in particular, that was the most widely used refrigerant at the time (R12, Freon-12 or Freon for short), was found to be a threat to the ozone layer which protects life from the harmful effects of the sun’s ultraviolet radiation, and even more so a contributor to global warming.
The class of refrigerants based on chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs) that includes R-12 and R-22 among others have been on a path toward the discontinuation of their use since a timeline was established by the Montreal Protocol. That timeline calls for the complete elimination of CFC and HCFCs by the end of this decade.
The common ingredient in both CFC and HCFC refrigerants is chlorine. Although the chlorine in these compounds is detrimental to the ozone layer, its effect (indicated by its ozone depletion potential or ODP) is of otherwise lower impact on the environment (apart from the ozone layer) than that of other commercially used synthetic refrigerants. Global warming potential (GWP) is of critical environmental consequence and many of these other refrigerants have significantly higher GWP numbers than that of CFC/HCFCs.
Following Montreal, Kyoto and more recently Paris, international agreements have built upon that initial effort to more substantially reduce and eliminate synthetic refrigerants. As part of this movement away from CHCs and HCFCs, another class of refrigerants, HFCs such as R134a were widely adopted. But HFCs were also found to be environmentally harmful as well.
International agreements have not been the only factor in the move away from synthetic refrigerants. Government regulation has played a significant part domestically in changing the industry. Here in the US, these include the Clean Air Act along with an alphabet soup of acronyms like EPA/SNAP, DOE, CARB and others. In Europe, EU F-gas regulations are yet another example. Regardless of where these regulations have been put in place however, the effect upon the industry has been profound.
Although the industry has responded with the search for environmentally safe synthetic alternatives, one of the most popular solution so far has been to initially limit the volume of these substances used such as through an approach called secondary loop systems, and then later through the eventual elimination of them entirely from the use by replacing them with natural refrigerants. This has resulted initially in glycol refrigeration systems which use a secondary loop approach. This approach limits the synthetic refrigerant to a small space where most of the refrigeration equipment in a store is housed.
Heat is transferred from the display cases and walk-ins to that part of the system through chilled loop piping that carries a propylene glycol solution which is a food-safe, environmentally friendly material.
More recently, with the arrival in the industry of carbon dioxide (CO2) first as another type of secondary fluid for low-temperature applications such as for frozen food door cases and walk-in freezers, and then later on as both a medium and low-temperature refrigerant through the development of
booster systems, the move toward entirely non-synthetic refrigeration has become considerably more widespread.
CO2 (R-744 in refrigerant nomenclature) is the benchmark for environmentally friendly, sustainable refrigeration against which practically all other refrigerants are measured. With a GWP of 1 as compared to R-22 (1810) and even R-410A (2088), it is so to speak a natural refrigerant choice. As of 2020, there are more than seven-thousand CO2 refrigeration systems installed worldwide with almost 700 of those in North America. CO2 refrigeration is clearly on the rise as more and more users across the entire range of food merchandising from convenience stores to supermarkets to warehouse clubs and distribution centers to large scale food processing and manufacturing operations are adopting it.
Distribution Center Application
Ammonia (R-717) widely used in industrial refrigeration actually has a lower GWP of 0. It is also highly toxic and as such ammonia refrigeration presently is not a practical solution for commercial applications. Whether ammonia (also known by its chemical name NH3) can one day be a practical solution to grocery store refrigeration remains to be seen.
Another innovative path toward sustainability has so far been employed primarily in grocery store display cases. Beginning in Europe and other foreign markets, propane (R-290) has proven beneficial when used as a refrigerant in single condensing units, the refrigeration components of stand-alone display cases. Domestically, more and more food retail applications are starting to make use of these. This is especially the case with smaller-format stores where room for large, centralized refrigeration systems may not be available. In those situations where space is at a premium, grocery store design can benefit from these types of display cases. With more and more R&D efforts from equipment manufacturers continuing, the eventual arrival of R-290 refrigeration systems is a possibility.
R290 Self-Contained Mobile Island Case
Given how long synthetic refrigerants have been in use, it’s not surprising that manufacturers and users alike are not entirely ready to abandon them. R-449A, an HFO refrigerant is one available solution. Whereas the change to most natural refrigerants, CO2 and propane for example, requires new equipment due primarily to the differences in their thermodynamic properties, refrigerants such as R449A offer the ability to continue to use it in systems designed for existing HFC refrigerants. HFOs typically have lower GWP numbers than HFCs and therefore allow users to meet current regulations even with equipment that was, for instance, originally using HCFCs like R-22.
The decision for any refrigeration system operator as to which refrigerant to choose is a complex one that requires multiple considerations to determine. The best way to come to that decision is to learn and understand as much as possible about those various factors not the least of which concern cost, sustainability and regulatory impacts.